Allogeneic Hematopoietic Stem Cell Transplant Market to Grow Rapidly During the Study Period (2020–2034) | DelveInsight
New York, USA, Oct. 21, 2024 (GLOBE NEWSWIRE) -- Allogeneic Hematopoietic Stem Cell Transplant Market to Grow Rapidly During the Study Period (2020–2034) | DelveInsight
The allogeneic hematopoietic stem cell transplant market is expected to experience significant growth in the coming years, driven by advances in transplantation techniques, improved donor-matching technologies, and a rising incidence of hematological malignancies. Increased awareness of the therapeutic potential of stem cell transplants, along with ongoing research into novel conditioning regimens and post-transplant care, will further boost market expansion.
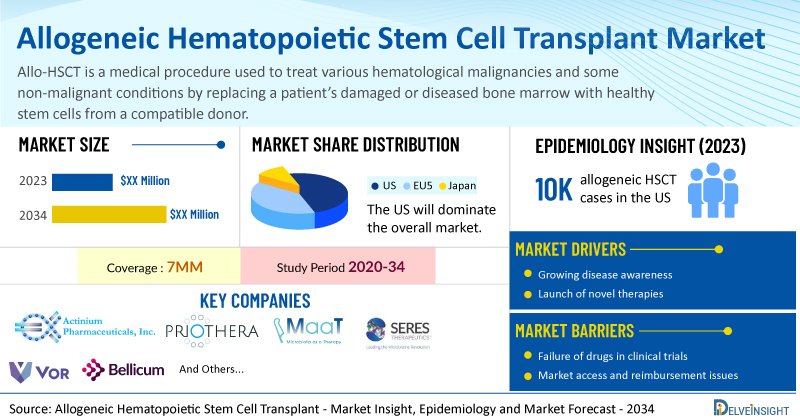
DelveInsight’s Allogeneic Hematopoietic Stem Cell Transplant Market Insights report includes a comprehensive understanding of current treatment practices, emerging allogeneic HSCT drugs, market share of individual therapies, and current and forecasted allogeneic HSCT market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Allogeneic Hematopoietic Stem Cell Transplant Market Report
- According to DelveInsight’s analysis, the market size of allogeneic HSCT in the 7MM is expected to grow at a significant CAGR by 2034.
- According to DelveInsight’s estimates, the United States accounted for the highest number of cases of allo-HSCT among the 7MM, in 2023.
- In 2023, the type-specific cases of HSCT in the United States were over 10,000 and 14,500 for allogeneic HSCT and autologous HSCT respectively.
- Prominent companies working in the domain of allogeneic HSCT, including Actinium Pharmaceuticals, Priothera, MaaT Pharma, Seres Therapeutics, Inc., Vor Biopharma, Bellicum Pharmaceuticals, and others, are actively working on innovative allogeneic HSCT drugs. These novel allogeneic HSCT therapies are anticipated to enter the allogeneic HSCT market in the forecast period and are expected to change the market.
- Some of the key allogeneic HSCT treatments include Iomab-B, Mocravimod, MaaT033, SER-155, VCAR33, VOR33, BPX-501, and others.
- Actinium Pharmaceuticals presented at the 3rd Annual Targeted Radiopharmaceuticals Summit US (July 30 – August 1, 2024), highlighting their lead asset, Iomab-B, for relapsed or refractory acute myeloid leukemia (AML).
- In November 2023, Priothera announced the US Food and Drug Administration (FDA) has granted Orphan Drug designation (ODD) to Mocravimod for the “treatment to improve outcome following hematopoietic stem cell transplantation in hematologic malignancies”
Discover which therapies are expected to grab the allogeneic HSCT market share @ Allogeneic HSCT Market Report
Allogeneic Hematopoietic Stem Cell Transplant Overview
Allogeneic hematopoietic stem cell transplant (allo-HSCT) is a medical procedure used to treat various hematological malignancies and some non-malignant conditions by replacing a patient’s damaged or diseased bone marrow with healthy stem cells from a compatible donor. This process involves several stages, including the conditioning regimen, which often involves chemotherapy and/or radiation to eliminate the diseased cells and suppress the immune system. The transplant itself can be performed using stem cells harvested from peripheral blood, bone marrow, or umbilical cord blood. The success of an allo-HSCT largely depends on the compatibility of the donor's cells with the recipient’s immune system, as well as the patient's overall health and disease status before the transplant.
Post-transplant, patients face several potential complications, the most significant being graft-versus-host disease, where the donor's immune cells attack the recipient's tissues. Despite these challenges, allo-HSCT can offer a curative option for patients with conditions such as leukemia, lymphoma, and certain inherited blood disorders. Advances in donor matching, improved supportive care, and enhanced immunosuppressive therapies have contributed to better outcomes and lower mortality rates associated with this complex procedure. Continuous research is underway to optimize allo-HSCT techniques and enhance patient safety and efficacy, making it a vital area of focus in hematology and oncology.
Allogeneic Hematopoietic Stem Cell Transplant Epidemiology Segmentation
The allogeneic HSCT epidemiology section provides insights into the historical and current allogeneic HSCT patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The allogeneic HSCT market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Cases of HSCT
- Total Cases of alllo-HSC
- Total Cases of allo-HSCT by Indications
Download the report to understand which factors are driving allogeneic HSCT epidemiology trends @ Allogeneic HSCT Epidemiological Insights
Allogeneic Hematopoietic Stem Cell Transplant Treatment Market
Hematopoietic stem cell transplantation has become a key treatment approach for patients with specific inherited or acquired blood-related disorders, as well as cancers sensitive to chemotherapy, radiotherapy, or immunotherapy. Over the past two decades, HSCT has seen rapid growth and continuous technological advancements. Conditions like multiple myeloma and non-Hodgkin's lymphoma are still the primary indications for high-dose chemotherapy followed by autologous peripheral blood stem cell transplantation.
OMISIRGE (omidubicel-onlv) is a modified allogeneic hematopoietic progenitor cell therapy, created from cord blood with nicotinamide alterations. It is approved for adults and pediatric patients aged 12 and older who have hematologic malignancies and are scheduled for umbilical cord blood transplantation after myeloablative conditioning. OMISIRGE aims to speed up neutrophil recovery and reduce infection rates. The FDA approved OMISIRGE for this indication on April 17, 2023, representing a major advancement in treating hematologic malignancies via cord blood transplantation.
TEPADINA (thiotepa) is an alkylating agent approved for allogeneic hematopoietic stem cell transplantation, especially in conditioning treatments for patients with blood cancers. It is produced by ADIENNE SA, a Swiss pharmaceutical company, and distributed in the U.S. by Amneal Pharmaceuticals. TEPADINA first received FDA approval in the U.S. in 1959, with a newer formulation approved on January 26, 2017. It has been approved for use in Europe since 2018 and in Japan since 2020. The drug is noted for its effectiveness in minimizing graft rejection and can be administered intravenously, intracavitary, or intravesically.
Learn more about the market of allogeneic HSCT @ Allogeneic HSCT Treatment
Allogeneic Hematopoietic Stem Cell Transplant Emerging Drugs and Companies
Some of the drugs in the pipeline include Iomab-B (Actinium Pharmaceuticals), Mocravimod (Priothera), and MaaT033 (MaaT Pharma) among others.
Actinium Pharmaceuticals is conducting a pivotal Phase III clinical trial for its lead asset, Iomab-B, as an induction and conditioning treatment for patients with relapsed or refractory AML before hematopoietic stem cell transplantation (HSCT). This therapy features an antibody that specifically targets CD45, which is commonly found in bone marrow cells, including cancer cells, and is linked to a radioactive isotope, iodine-131.
The US FDA and EMA have granted Iomab-B Orphan Drug Designation for patients aged 55 and older with relapsed or refractory AML. In February 2023, Actinium announced that Iomab-B achieved remarkable 100% access to bone marrow transplantation and engraftment, meeting the primary endpoint of the SIERRA trial. The company plans to submit a Biologics License Application (BLA) in 2024 to seek approval for Iomab-B for patients aged 55 and older with relapsed or refractory AML who lack access to current therapies for bone marrow transplantation.
Mocravimod is a new synthetic modulator of sphingosine 1-phosphate receptors (S1PR) being studied as an additional and ongoing treatment in the Phase IIb/III trial known as MO-TRANS for AML patients undergoing allo-HSCT. It has been granted Fast Track designation by the FDA and Orphan Drug status in both the US and Europe. The goal of Mocravimod is to sustain anti-leukemia effects while minimizing the risk of graft-versus-host disease.
MaaT033 is an oral therapy derived from donor microbiomes, designed to provide a standardized, rich, and diverse microbiome ecosystem for patients undergoing allo-HSCT. It has been granted Orphan Drug Designation by the EMA. The therapy aims to restore gut microbiome diversity and maintain immune balance to prevent infections and graft-versus-host disease. A Phase Ib trial demonstrated effective microbiome engraftment and safety, and a Phase IIb trial named PHOEBUS is currently underway to evaluate its efficacy in enhancing overall survival at 12 months.
The other pipeline therapies for allogeneic HSCT include
- SER-155: Seres Therapeutics, Inc.
- VCAR33: Vor Biopharma
- VOR33: Vor Biopharma
- BPX-501: Bellicum Pharmaceuticals
The anticipated launch of these emerging therapies are poised to transform the allogeneic HSCT market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the allogeneic HSCT market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about allogeneic HSCT clinical trials, visit @ Allogeneic HSCT Treatment Drugs
Allogeneic Hematopoietic Stem Cell Transplant Market Dynamics
The allogeneic HSCT market dynamics are anticipated to change in the coming years. HSCT is the only potentially curative treatment for patients with chemotherapy-resistant hematological malignancies, which are usually fatal without treatment, and it is a widely accepted therapeutic modality for various malignant, hematologic, immunologic, and genetic diseases; this therapy involves the intravenous infusion of hematopoietic progenitor cells to reestablish marrow function in patients with damaged or defective bone marrow, with improved outcomes attributed to advancements in tissue typing, prophylaxis against viral and fungal infections, immunosuppressive drugs, and supportive care, while recognizing risk factors for complications enables the design of risk-specific supportive-care regimens that help reduce the incidence of transplantation morbidity and mortality.
Furthermore, many potential therapies are being investigated for the treatment of allogeneic HSCT, and it is safe to predict that the treatment space will significantly impact the allogeneic HSCT market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the allogeneic HSCT market in the 7MM.
However, several factors may impede the growth of the allogeneic HSCT market. HSCT is associated with both immediate and long-term complications that require increased vigilance and monitoring; these complications can lead to decreased quality of life and shortened life expectancy. The high chemotherapy doses used in HSCT result in significant drug toxicities and complications from prolonged immunodeficiency, necessitating an extended recovery process. HSCT also entails high costs and demands highly specialized medical infrastructure and a network of specialists across various fields. Furthermore, mortality rates in HSCT can be influenced by comorbidities, disease characteristics, HLA matching, GvHD, the graft-versus-tumor effect, and post-transplant recurrence, with relapse-related mortality reflecting tumor biology, non-relapse mortality from HSCT procedures, and patient comorbidities.
Moreover, allogeneic HSCT treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the allogeneic HSCT market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the allogeneic HSCT market growth.
| Allogeneic Hematopoietic Stem Cell Transplant Report Metrics | Details |
| Study Period | 2020–2034 |
| Allogeneic Hematopoietic Stem Cell Transplant Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Allogeneic Hematopoietic Stem Cell Transplant Companies | Actinium Pharmaceuticals, Priothera, MaaT Pharma, Seres Therapeutics, Inc., Vor Biopharma, Bellicum Pharmaceuticals, and others |
| Key Allogeneic Hematopoietic Stem Cell Transplant | Iomab-B, Mocravimod, MaaT033, SER-155, VCAR33, VOR33, BPX-501, and others |
Scope of the Allogeneic Hematopoietic Stem Cell Transplant Market Report
- Allogeneic Hematopoietic Stem Cell Transplant Therapeutic Assessment: Allogeneic Hematopoietic Stem Cell Transplant current marketed and emerging therapies
- Allogeneic Hematopoietic Stem Cell Transplant Market Dynamics: Conjoint Analysis of Emerging Allogeneic Hematopoietic Stem Cell Transplant Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Allogeneic Hematopoietic Stem Cell Transplant Market Access and Reimbursement
Discover more about allogeneic HSCT in development @ Allogeneic HSCT Clinical Trials
Table of Contents
| 1. | Allogeneic Hematopoietic Stem Cell Transplant Market Key Insights |
| 2. | Allogeneic Hematopoietic Stem Cell Transplant Market Report Introduction |
| 3. | Allogeneic Hematopoietic Stem Cell Transplant Market Overview at a Glance |
| 4. | Allogeneic Hematopoietic Stem Cell Transplant Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Allogeneic Hematopoietic Stem Cell Transplant Treatment and Management |
| 7. | Allogeneic Hematopoietic Stem Cell Transplant Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Allogeneic Hematopoietic Stem Cell Transplant Marketed Drugs |
| 10. | Allogeneic Hematopoietic Stem Cell Transplant Emerging Drugs |
| 11. | Seven Major Allogeneic Hematopoietic Stem Cell Transplant Market Analysis |
| 12. | Allogeneic Hematopoietic Stem Cell Transplant Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Allogeneic Hematopoietic Stem Cell Transplant Epidemiology Forecast
Allogeneic Hematopoietic Stem Cell Transplant Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted allogeneic HSCT epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Hematopoietic Stem Cell Transplantation Pipeline
Hematopoietic Stem Cell Transplantation Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key HSCT companies, including Actinium Pharmaceuticals, BioLineRx, Athersys, Novartis, CareDex, Orchard Therapeutics, Magenta Therapeutics, Graphite Bio, Vor Biopharma, Jasper Therapeutics, Garuda Therapeutics, among others.
Allogeneic Hematopoietic Stem Cell Transplant Market
Allogeneic Hematopoietic Stem Cell Transplant Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key HSCT companies including Merck Sharp & Dohme LLC, In8bio Inc., Incyte Corporation, Forge Biologics, Inc, GlaxoSmithKline, Bellicum Pharmaceuticals, SecuraBio, ModernaTX, Inc., Miltenyi Biotec, Inc., Jazz Pharmaceuticals, Gilead Sciences, Celularity Incorporated, Seres Therapeutics, Inc., Omeros Corporation, Novartis, Marker Therapeutics, Inc., among others.
Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key multiple myeloma companies, including Johnson & Johnson (Janssen), Pfizer, AbbVie and Roche (Genentech), Regeneron Pharmaceuticals, Bristol-Myers Squibb, Celgene, Roche (Genentech), Arcellx, Novartis, Regeneron Pharmaceuticals, BeiGene, CARsgen Therapeutics, Cartesian Therapeutics, C4 Therapeutics, Heidelberg Pharma, Bristol-Myers Squibb, RAPA Therapeutics, AbbVie (TeneoOne), Takeda, among others.
Non-Hodgkin’s Lymphoma Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NHL companies, including Novartis, AstraZeneca, Genentech, BioInvent, Genmab, SystImmune, Nordic Nanovector, Pacylex Pharmaceuticals, Artiva Biotherapeutics, Inc., Chipscreen Biosciences, Ltd., Timmune Biotech Inc., Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Gilead Sciences, Acerta Pharma BV, Adagene Inc, Conjupro Biotherapeutics, Inc., Rhizen Pharmaceuticals, Juventas Cell Therapy Ltd., Incyte Corporation, HUYA Bioscience International, SecuraBio, Genor Biopharma Co., Ltd., Kyowa Kirin Co., Ltd., Antengene Therapeutics Limited, Regeneron Pharmaceuticals, Jiangsu HengRui Medicine Co., Ltd., Xynomic Pharmaceuticals, Inc., BioTheryX, Inc., UWELL Biopharma, Kronos Bio, Bio-Thera Solutions, Spectrum Pharmaceuticals, Inc., Aptose Biosciences Inc., Miltenyi Biomedicine GmbH, Precision BioSciences, Inc., Teneobio, Inc., TCR2 Therapeutics, IGM Biosciences, Inc, among others.
Best-in-class Business Consulting Services
Healthcare Conference Coverage
Healthcare Competitive Intelligence
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
© Copyright Globe Newswire, Inc. All rights reserved. The information contained in this news report may not be published, broadcast or otherwise distributed without the prior written authority of Globe Newswire, Inc.


